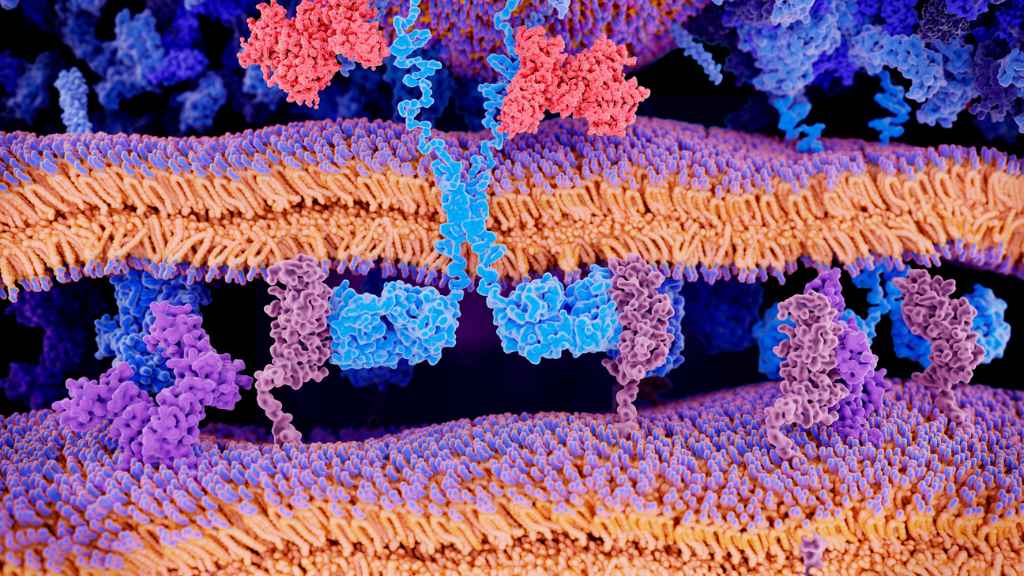
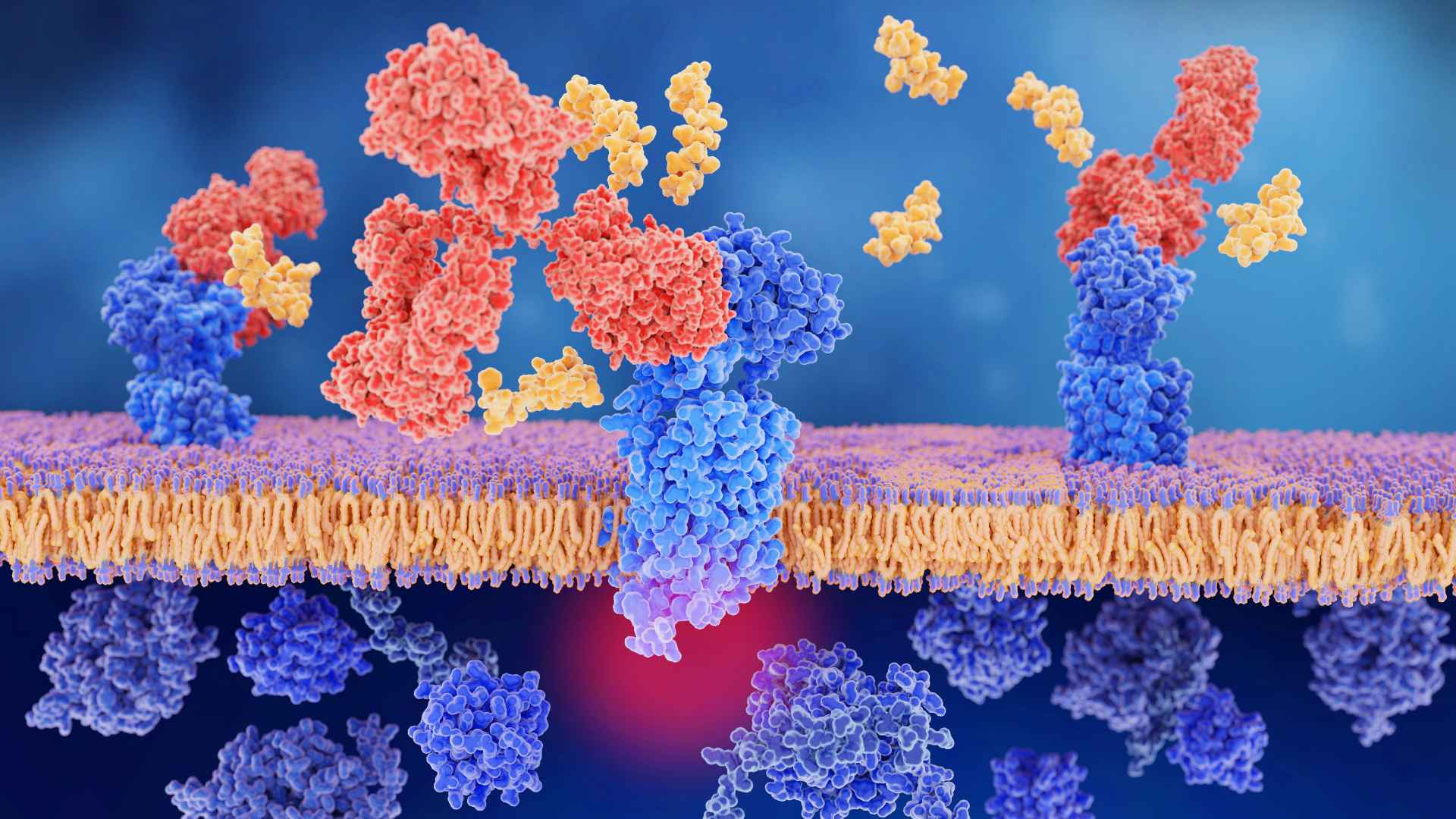
Picture a bustling city at rush hour—every person is a protein, and each interaction they have forms the living, breathing entity of our cells. This isn’t science fiction; it’s the reality of protein interaction. It’s like being in the heart of Times Square where every handshake and conversation can change lives—or in this case, determine how our bodies function.
Maybe you’ve heard bits about proteins linking up like dancers in an elaborate ballet. Well, stick around because we’re diving deep into that dance floor to see who leads, who follows, and what happens when someone steps on another’s toes.
We’ll uncover secrets hidden within molecular embraces: from cell growth to fighting diseases. And trust me—the implications are huge. Ready for a sneak peek into life’s microscopic social network? Let’s get started!
Understanding Protein-Protein Interactions and Their Networks
Imagine a bustling city where everyone has an essential role. That’s kind of what it’s like inside our cells, with proteins interacting in complex networks to keep things running smoothly. These protein-protein interactions (PPIs) are the cellular equivalent of social networking—except instead of sharing cat videos, they’re swapping crucial biological information.
The Role of Protein Complexes in Cellular Function
In this vast network, think of protein complexes as the high-powered meetings that decide how a cell behaves. They call the shots on everything from cell growth to gene expression—a real corporate ladder within our biology. And we’re not talking about just a few proteins here; there are over 59 million known proteins playing tag across more than 12,535 organisms—and those numbers are growing.
But these aren’t random encounters; specific biological rules govern who talks to whom or which proteins interact with each other. It’s like an exclusive club where only members with the right molecular weight or amino acid sequence can enter.
Digging deeper into these interaction studies reveals stable interactions—the long-term relationships—that maintain cellular processes including all-important gene expression pathways. On the flip side, transient interactions act like speed dating events: quick but vital meet-ups that lead to short-lived yet significant effects on biological processes.
STRING Database, a treasure trove for PPI enthusiasts, offers insights into over 20 billion such connections. Here you’ll find abundant proteins casually mingling at any given time and rarer ones making guest appearances—all crucial players in life’s grand scheme.
Comprehensive Methods for Studying Protein-Protein Interactions
To map out these extensive networks accurately requires Sherlock-Holmes-level detection methods—because sometimes observing PPIs is as tricky as spotting Bigfoot at a crowded concert. In vitro techniques allow scientists to pull apart these elusive binding partners using affinity purification followed by mass spectrometry—a duo that can spot even faint whispers between two interacting molecules.
In vivo approaches offer another angle—they let us observe this molecular dance floor directly within living cells using methods akin to setting up hidden cameras—in scientific terms called yeast two-hybrid systems—to catch proteins red-handed as they cozy up together naturally.
If detective work had Olympic games, crosslinking protein interaction analysis label transfer would be taking home gold medals for its ability capture fleeting glances between shy molecules too bashful for traditional assays—an indispensable tool when it comes down studying dynamic webs interconnections define how alive functions moment-to-moment basis
Remember though—even while armed with these insights, you’ll still need to stay agile and responsive to shifts in market trends. Keep an eye on customer feedback and industry updates. They can offer valuable clues that help your business adapt effectively.
Comprehensive Methods for Studying Protein-Protein Interactions
In Vitro Techniques for PPI Detection
Picture this: a molecular meet-and-greet where the who’s who of proteins mingle. That’s essentially what happens in affinity purification, one of several in vitro methods to detect protein-protein interactions (PPIs). By baiting specific proteins with their known partners, scientists can observe and analyze these complex relationships like matchmakers studying potential couples at a speed dating event. It’s not just about catching two proteins holding hands; it involves identifying entire networks that might be as intricate as your last family reunion.
If affinity purification is the social mixer, then mass spectrometry is like having a guest list with everyone’s name and details. Proteins don’t wear nametags, but they do have unique molecular weights which act as their identification badge when we run them through SDS-PAGE gels after the pull-down assay has worked its magic. Imagine being able to tell who danced with whom simply by weighing their shoes – that’s how precise this technique gets.
The blot analysis adds another layer to our investigation – think of it as capturing photographic evidence of those fleeting moments between two interacting proteins before they part ways again. And let’s not forget Western blots; if you ever want proof beyond reasonable doubt that protein A met protein B at some point during the night, this method will give you that clear band on an x-ray film akin to finding lipstick on a collar.
In Vivo Approaches to Study Proteins in Their Natural Context
Diving into living cells offers us front-row seats at nature’s greatest show—proteins performing live. The yeast two-hybrid system lets us peek behind cellular curtains where transient interactions occur faster than celebrity romances tabloids love so much.
This technique doesn’t stop there though—it helps unravel even stable interactions akin to old married couples comfortable in each other’s presence over time without drama or fanfare. With label transfer techniques added into the mix, scientists now hold VIP backstage passes allowing them access all areas within these cellular concerts – observing every handshake and high-five shared among proteins acting out life-sustaining processes including gene expression control mechanisms inside cells bustling busier than Grand Central Station during rush hour.
STRING Database, reveals there are more than 20 billion such liaisons among nearly 60 million players across over 12 thousand organisms. Now if only keeping track of our own personal connections were half as systematic.
With technology progressing faster than anyone shouting “Eureka.” in labs worldwide—we’ve got ourselves tools that are revolutionizing the way we work, communicate, and live our daily lives. It appears as if every day brings a new device or software program aiming to surpass the prior one. And honestly? We’re here for it.
Advanced Detection Methods for Unraveling Protein Interaction Dynamics
When it comes to understanding the lifelines of cellular processes, protein interactions steal the spotlight. But how do scientists untangle this web? Let’s just say that with over 59.3 million proteins engaging in more than 20 billion interactions across various organisms, traditional methods won’t cut it.
The Role of Crosslinking Protein Interaction Analysis Label Transfer
Detection methods have evolved from simple observation to a sophisticated dance of technology and biology. Among these, crosslinking protein interaction analysis label transfer (CPIALT) stands out as a Sherlock Holmes in our molecular mystery – piecing together transient and stable liaisons between proteins like puzzle pieces fitting perfectly into place.
This technique goes beyond mere identification; it offers snapshots of proteins caught in action. Picture this: two interacting proteins are ‘frozen’ by chemical linkers which act as handcuffs at their point of contact – CPIALT then steps in to identify who was holding hands when the music stopped.
Decoding Interactions Biological Significance with Tandem Affinity Purification
Moving on from capturing moments is tandem affinity purification (TAP), an approach not unlike social media stalking but for proteins – following them around to see who they hang out with and thus revealing their biological cliques or complexes within cells. This method can help us understand why certain groups come together under specific conditions, impacting everything from gene expression to cell growth.
TAP gives us intel on those abundant proteins that might be hogging all the attention—like RNA polymerase—and helps decipher what makes them so popular among other biomolecules within cellular processes including transcription regulation and DNA repair pathways.
Navigating Through Proteins Using Mass Spectrometry Like A GPS System
If you’ve ever used a GPS system, mass spectrometry isn’t too different when navigating through proteomic landscapes. It sizes up molecules based on weight—a sort of ‘molecular weigh station’ if you will—allowing researchers to zero in on target proteins without getting lost amid millions others dancing about inside cells.”
European Molecular Biology Laboratory’s website serves as one such compass providing valuable resources for researchers charting unknown territories.
We’re talking cutting-edge detection here. With mass spec’s ability we get real-time updates on amino acid sequences constructing our beloved actors—the single protein participants involved intimately behind-the-scenes where life’s drama unfolds onstage daily.”
Tandem Affinity Purification: Fishing Out Abundant Proteins From Crowds
Tandem Affinity Purification (TAP) is a powerful technique used to isolate and identify protein complexes from complex mixtures. It involves the use of a protein tag that allows for the purification of the protein of interest along with its interacting partners.
The TAP method typically involves two steps. In the first step, the protein of interest is tagged with two different affinity tags, such as a protein A tag and a protein C tag. The tagged protein is then expressed in cells and lysed to release the protein complex.
In the second step, the lysate is subjected to affinity purification using two different affinity matrices. The first affinity matrix contains a resin that specifically binds to the protein A tag, allowing for the purification of the protein of interest and its associated partners. The eluted proteins are then subjected to a second round of purification using a resin that specifically binds to the protein C tag.
The TAP method allows for the purification of protein complexes in a highly specific manner, as the presence of both affinity tags ensures that only the desired protein complex is isolated. This method has been utilized extensively to examine protein-protein interactions and has yielded important knowledge into the structure and role of protein complexes in various biological activities.
The Biological Implications of Protein-Protein Interaction Networks
Now, replace the people with proteins and you’ve got yourself a glimpse into the cellular world. It’s not just about who’s there; it’s how they interact that shapes life as we know it.
The Role of Protein Complexes in Cellular Function
A single protein is like one piece of a jigsaw puzzle—it only gives part of the picture. When proteins bind together to form complexes, they create vibrant snapshots essential for cell function. These interactions are crucial for everything from replicating DNA to responding to external signals—basically all biological processes including gene expression hinge on this dynamic dance.
Consider an orchestra: Each musician (protein) may be stellar solo but put them together and you get symphonies (biological effects). In our bodies, these ‘symphonies’ ensure cells behave correctly—like growing when needed or fighting off invaders.
Comprehensive Methods for Studying Protein-Protein Interactions
Digging into protein-protein interaction networks requires detective work using tools like affinity purification or mass spectrometry—a bit like forensic experts at a crime scene piecing together clues left behind by interacting proteins. For those preferring live-action over lab tales, techniques such as yeast two-hybrid systems let us spy on protein rendezvous right where they happen—in living cells.
Advanced Detection Methods for Unraveling Protein Interaction Dynamics
Catch transient interactions—the ones that play hard-to-get—is tough business. But thanks to advanced methods involving crosslinking protein interaction analysis label transfer, scientists can now freeze-frame these fleeting flings between proteins providing invaluable insight into their complex lives.
Key Factors Influencing Protein-Protein Interactions
You might think size matters in protein relationships—but actually, it’s more about shape and chemistry. The amino acid sequence dictates structure while post-translational modifications act like relationship counselors adjusting attraction strength between binding partners. Think speed dating meets molecular biology.
Dynamics of Target Proteins Within Interaction Networks
In this intricate network lies target proteins—the social butterflies connecting with multiple partners affecting diverse functions within our cells ranging from metabolism boosters to gatekeepers regulating what goes in and out. This mingling affects not just individual pathways but whole cellular ecosystems because every handshake leaves an imprint on life itself. Imagine your local barista knowing everyone’s coffee order—that’s your target protein at work making sure everyone gets exactly what they need when they need it.”
The data clearly shows why we need to focus on PPIs—organisms carry millions of proteins that interact in complex ways. Comprehending these interconnections can bring about groundbreaking advances in medicinal and pharmaceutical progress.
Dynamics of Target Proteins Within Interaction Networks
Imagine a dance floor where proteins are the dancers, and each move they make is crucial to life’s choreography. That’s your cell on a typical day, buzzing with target proteins that don’t just groove solo; they’re all about those partner moves. But what happens when these partners link up?
The Role of Protein Complexes in Cellular Function
Let’s break it down: protein complexes aren’t just hanging out together for fun. They’re pivotal players orchestrating cellular processes smoother than an orchestra conductor waving their baton. Picture over 59 million proteins across more than 12 thousand organisms—yeah, we’ve got numbers like a cosmic census—all engaging in over 20 billion interactions that dictate everything from cell growth to gene expression.
It’s like every protein complex has its own social network profile on STRING Database, flaunting who it hangs with and how often. These profiles give us the scoop on how cells tick—the who’s who of biological networking.
Intricate Interactions Amongst Abundant Proteins
If you think keeping track of one relationship is hard, try juggling multiple at once. Target proteins are smooth operators linking up with various interacting partners faster than speed daters at a cafe. Their stability isn’t just for show either; forming stable interactions means locking down functions vital for survival while transient interactions allow them quick flings – swapping partners as needed without getting tied down.
We’ve got tools sharper than kitchen knives to suss out these connections too—like crosslinking protein interaction analysis label transfer techniques—that tag team to reveal even the most fleeting romances between molecules within this bustling singles’ bar we call the cytoplasm.
Navigating Through Molecular Weight and Amino Acid Mazes
Every now and then though, size does matter—in terms of molecular weight anyway—and not just because some gym rat said so but because it influences which heavyweight or lightweight gets into the ring next. And amino acids? Think of them as personality traits influencing attraction levels among our protein pals; specific sequences lead to sparks flying—or fizzling—in this microscopic dating game.
Finding Patterns in Chaos: The Dance Moves Decoded
You know those folks good at reading people? Well, scientists can read patterns amidst chaotic cellular dances by analyzing things like SDS-PAGE gels—a technique sorta akin to checking footprints left behind after a wild dance-off—to determine who danced with whom based on residual moves imprinted onto gel stages.

Tackling Challenges in PPI Analysis with Innovative Techniques
Studying protein-protein interactions (PPIs) is like trying to listen to every conversation at a bustling party. You want to know who’s talking to whom, what they’re saying, and why it matters—no easy task when the room’s buzzing with over 59 million proteins chatting away. Imagine tuning into these dialogues that influence cellular processes from gene expression to cell growth; this is the daily hustle of scientists working on PPI analysis.
Innovative Approaches for Protein Interaction Analysis
Let’s cut through the noise of traditional methods and zoom in on label transfer techniques that have shaken things up in the realm of interaction studies. These clever tactics tag along with proteins as they mingle, offering insights by marking those brief handshakes and long-term partnerships within cells. Picture someone wearing a bright neon hat at our crowded party—it’s hard not to notice them or see who they’re hanging out with. That’s how label transfer lights up interacting proteins.
The same goes for crosslinking protein interaction analysis—a method that acts like introducing superglue into conversations, locking participants together just long enough for us to take note before everyone moves on. It gives researchers snapshots of transient interactions often missed by other approaches.
Pioneering Detection Methods Unveiling Dynamic Interactions
Gone are the days when detection was limited by molecular weight or protein sequence obscurity. Now we’ve got SDS-PAGE gel electrophoresis combined with mass spectrometry allowing us an all-access pass behind closed doors where stable interactions form complex networks essential for life itself—and we can even peek at single-protein subtleties without disturbing their natural habitat.
Dive deeper than ever before using tandem affinity purification which brings forth abundant proteins such as RNA polymerase into plain view amidst biological effects too intricate for less discerning eyes—like spotting a rare bird among common pigeons thanks EMBL research teams’ dedication.
Navigating Through Cellular Mazes: In Vivo vs In Vitro Perspectives
In vivo techniques tease apart layers revealing not just bare bones but fleshed-out stories about each character within cellular dramas playing across organisms counted beyond twelve thousand—with strings attached indeed. Enter STRING Database, your guidebook listing whos-who in biological blockbusters involving more than twenty billion PPIs depicted so far.
But let’s not overlook vitro virtuosos spinning tales straight from the lab, weaving complex science into stories we can all relate to.
The Evolutionary Perspective on Protein-Protein Interactions
Proteins involved in the dance of life don’t groove alone. They pair up, forming wide-ranging interactions that are crucial to every jig and jive within our cells. But ever wonder how this complex choreography came to be? It’s a story written in the annals of evolution.
Decoding the Conservation and Divergence Dance
Astonishingly, across 12,535 organisms there’s a record-breaking number—59.3 million proteins—and they’ve got history. More than 20 billion protein-protein interactions (PPIs) have been documented so far, showing us just how conserved some steps of this molecular tango really are. By peering into these vast networks through resources like STRING Database, we catch glimpses of ancient moves passed down through millennia.
Digging deeper into evolutionary studies reveals an intricate pattern: while many PPIs remain unchanged over time, others branch out with new flair—a divergence adding complexity to life’s rhythms. These patterns show us not only which proteins interact but also give clues about why certain duos stick together throughout different species.
Tuning Into Nature’s Frequency – The Role of Amino Acids and Molecular Weight in PPI Harmony
In tuning their instruments for interaction analysis label transfer experiments help identify partners based on amino acid sequences and molecular weight tags—it’s like recognizing musicians by their instrument sounds. Yet amid this orchestra lie transient interactions; brief encounters akin to improvisational jazz solos that nonetheless influence cellular processes profoundly.
Consider crosslinking protein interaction analysis label transfer methods as nature’s way of hitting ‘record’ during live sessions—capturing fleeting moments where proteins briefly touch before parting ways again—revealing who brushed past whom even if it was just for a second.
Finding Stability Amidst Cellular Chaos – Unveiling Stable Interactions Biological Significance
If transient interactions are quick sparks between two entities then stable interactions form solid partnerships built over countless rehearsals giving rise to biological effects beyond mere gene expression control—they shape entire biological landscapes.
Analyzing these relationships can feel like reading sheet music full of dynamic marks—the crescendos representing strong binding partners while pianissimos hint at subtler liaisons—all playing roles essential for cell growth harmony or discord depending on context; such is nature’s composition revealed via affinity purification techniques among other tools at hand when delving into interaction studies involving abundant proteins like RNA polymerase which dictate tempo from behind-the-scenes.
The Future Landscape of PPI Research and Its Potential Impact
Picture a world where understanding the handshake between proteins can unlock cures for diseases that baffle us today. That’s the potential future we’re looking at with protein-protein interaction (PPI) research taking center stage in biomedical science.
Challenges and Future Directions in Protein Interaction Analysis
Advancements in PPI analysis are not just anticipated; they’re needed, pronto. Current methods give us snapshots of these intricate dances but imagine capturing every twirl and dip. We face challenges like untangling transient interactions from stable ones—akin to picking apart two dancers mid-jive—but innovative techniques are on the horizon to make this possible. Imagine if we could zoom into molecular weight differences or amino acid nuances as easily as scanning through a playlist—it would be game-changing.
New tech means fresh ways to look at old problems, right? So let’s chat about how things like label transfer protein interaction analysis could revamp our viewfinder. With this approach, you tag your target proteins like putting a tracking device on an elusive animal—you get data that helps you predict their next move. It’s high-stakes hide-and-seek where finding binding partners equals major scientific wins.
Tackling Modern Analytical Techniques Head-On
Diving deeper into detection methods feels like arming ourselves with superpowered goggles—we see what was once invisible. Crosslinking protein interaction studies now give insights into which proteins cozy up under cellular stress or during different stages of cell growth—a bit like social media for cells, revealing who hangs out with whom at various events.
We’ve got more than one trick up our sleeve though; SDS-PAGE gels have long been used by scientists everywhere—and I mean everywhere—with over 12,535 organisms’ worth of data showing off 59.3 million unique proteins engaged in more than 20 billion interactions logged so far STRING Database. The challenge is evolving from simply naming these abundant proteins to really getting why and how they interact within biological processes including gene expression—because isn’t knowing ‘why’ always better?
A Glance Through Evolutionary Lens at PPIs
Peering back across time gives context because even something as complex as RNA polymerase has ancient roots—the basics don’t change much even when life diversifies wildly around them. Our current work may focus on specific biological systems or conditions but connecting those dots across evolutionary timelines offers robust clues about function preservation—or divergence—that can direct future research efforts toward meaningful breakthroughs.
Let’s launch into the future and make something awesome. Let’s get ready to make things happen.
Conclusion
Think of each protein interaction as a vital conversation in the cell’s bustling metropolis. These interactions shape our health, whispering life into every biological process.
Remember those methods we explored? They’re your eyes and ears on this dance floor—crucial for catching every subtle gesture between proteins.
Hold onto how structures and modifications influence who talks to whom. It’s key for understanding cellular chatter.
Dive deeper with these insights; they’re tools to unlock secrets of diseases, guiding us toward novel treatments. Protein interaction isn’t just a concept—it’s the pulse that drives our living cells forward.
If you’re set on grasping life at its most fundamental level or crafting therapies of tomorrow, let this be your stepping stone into the intricate world where proteins interact.